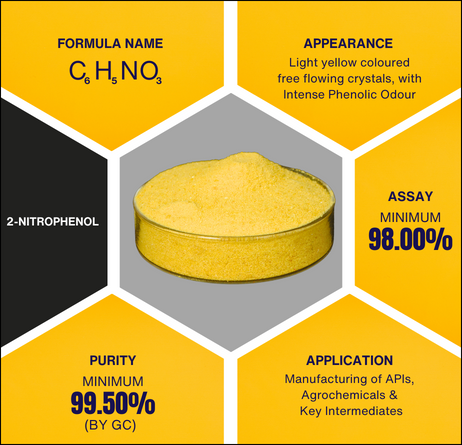
2-Nitrophenol
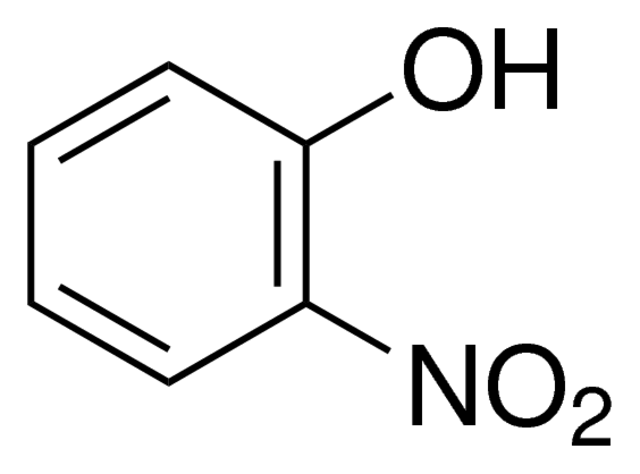
2-Nitrophenol, which is also called o-nitrophenol, ortho nitrophenol, or ONP, is an important member of the phenol family. In this family, a hydroxyl group (-OH) is directly attached to a benzene ring. The o nitrophenol formula, C6H5NO3, is another name for this chemical formula. The ortho-positioned nitro group (-NO2) in relation to the hydroxyl group is what makes this molecule stand out. The ortho nitro phenol structure is what provides its own distinct physical, chemical, and reactive features.
The color of 2-nitrophenol is pale yellow and it forms free-flowing crystals, which is typical of ortho nitrophenol. The hydroxyl and nitro groups in the structure of o nitrophenol interact with one another, making the substance a little acidic.
Chemical Information
- Chemical Name: 2-Nitrophenol
- Other Names: o-Nitrophenol, Ortho Nitro Phenol, ONP
- Chemical Formula: C₆H₅NO₃
- CAS Number: 88-75-5
- EC Number: 210-393-2
- Molecular Weight: 139.11 g/mol
- Assay (HPLC): Minimum 98.00%
- Chlorides as NaCl: Maximum 0.50%
- Moisture: Maximum 0.50%
- Appearance: Light yellow crystalline powder
- Melting Point: Approx. 45°C
- Boiling Point: Approx. 213°C
- Solubility: Slightly soluble in water; soluble in ethanol, ether
- Packaging: 25 kg net weight in HDPE woven bags with inner polyliner
Applications
Pharmaceuticals and Medical Applications
Uses 2-Nitrophenol derivatives are widely utilized in the pharmaceutical industry for medical and pharmaceutical purposes. They are important for making intermediates that have antibacterial, anti-inflammatory, and pain-relieving effects. Some derivatives have also shown promise as cancer-fighting drugs that kill cells. It is easy to add functional groups to nitrophenol because of its structure. This is important for making and designing drugs.
Dye and Pigment Industry
2-Nitrophenol serves as an intermediate compound in the production of dyes and pigments. Its reactivity allows for the introduction of additional functional groups, resulting in a wide range of vibrant and long-lasting colorants used in textiles, plastics, and other materials.
Agrochemical Industry
Even though 2-nitrophenol is not an agrochemical itself, its nitro and hydroxyl groups in the ortho position are important for making pesticides, fungicides, and herbicides. Agrochemical researchers can make compounds with specific bioactivity by changing the structure of 2-nitrophenol.
Environmental Significance
o-Nitrophenol is a common contaminant that is often made as a by-product of making drugs, dyes, and insecticides in factories. Because o nitrophenol stays in the environment for a long time, knowing its structure can help scientists come up with new ways to clean up pollution and manage wastewater.
Chemical Intermediates
The ortho nitrophenol framework makes it an excellent precursor in organic synthesis. It is used in the production of:
- Rubber accelerators
- Antioxidants
- Corrosion inhibitors
- UV absorbers
- Fine chemicals and lab reagents
Its o nitrophenol formula offers compatibility with a range of chemical reactions—making it a critical building block in industrial chemistry.

Comparison: 2-Nitrophenol vs. 4-Nitrophenol
| Feature | 2-Nitrophenol (o-Nitrophenol) | 4-Nitrophenol (p-Nitrophenol) |
|---|---|---|
| Nitro Group Position | Ortho | Para |
| Melting Point | ~45°C | ~113°C |
| Boiling Point | ~213°C | ~279°C |
| Acidity | More acidic | Less acidic |
| Colour | Light yellow (ortho nitrophenol colour) | Pale yellow / light brown |
| Applications | Dyes, Pharma, Intermediates | Paracetamol synthesis, Indicators |
Packaging Options
Domestic Packaging
- 25 kg Fibre Drums with inner liner
- 50 kg HDPE woven bags with LDPE liner for extra protection


Conclusion
2-Nitrophenol (also known as o-nitrophenol or ortho nitrophenol) is a versatile industrial compound. Its characteristic ortho nitro phenol structure makes it indispensable in pharmaceuticals, dyes, agrochemicals, and chemical intermediates. Though valuable, it also poses environmental concerns, which necessitates responsible usage and sustainable handling. Understanding the structure of o nitrophenol continues to drive research across various industries, ensuring that its full potential is tapped while mitigating ecological impact.
